Hops: Anatomy and Chemistry 101

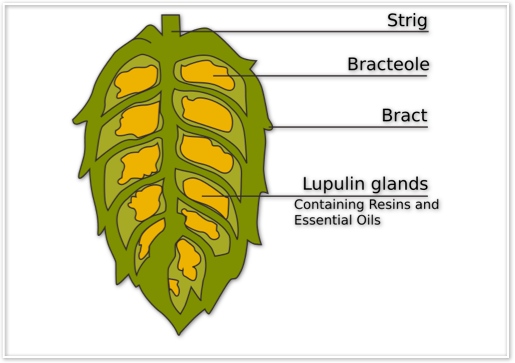
As seen in the figure, the cone is very simply made up. Bracts, and bracteoles (small bracts) are leaf like structures that surround the entire cone, attaching to a central axis. Underneath the bracteoles is where the magic of the hop plant is found, the lupulin glands. Lupulin glands contain the resins and oils most brews are looking for when making beer. Although a multitude of chemicals are present in these glands, the three main ones are alpha and beta acids, along with essential oils. These three chemicals are very important in the making of modern beer.
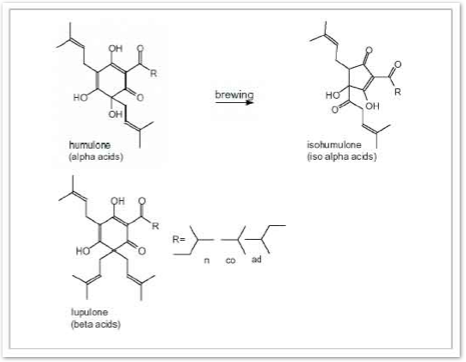
Beta acids:
Primary Beta acids:
=> Lupulone
=> Colupulone
=> Adlupulone
Beta acids (BA) do not contribute much to the beer as a whole, but help in two areas. First, beta acids contribute some to the bitter flavor of the beer. Although the bitterness from the beta acids are harsher than the alpha acids, due to their insolubility, not much comes off in the beer. The beta acids do not isomerize like there alpha counter part, but rather oxide in the beer to produce bitter flavor. Their affect is seen over the long-term, becoming more developed with long fermentation, storage, and aging. Dry hopping, the process of adding hops to the fermenting vessel without any other preparation, is another way beta acids are brought into the beer.
BA have also been shown to have great antiseptic qualities for the beer, aiding in shelf life and yeast’s ability to grow. Some research has been done using BA from H. lupulus to aid in the preservation of foods.
Alpha acids:
Primary Alpha Acids:
=> Humulone
=> Cohumulone
=> Adhumulone
Alpha acids (AA) have two main purposes in beer. First, they give beer that unique bitter taste. Most of the alpha acids are transferred into the wort (this is the fancy term for the sweet liquid that comes from boiling grains; basically this is unfermented beer) gradually over the amount of time they are boiled. The longer the hop is boiled while brewing, the more alpha acid is transformed into iso-alpha acids, a more soluble form and compound that gives beer that distinct bitter taste. To maximize the amount of alpha acids to iso-alpha acids, the hops used are put in the boil very early, usually being kept in for 60 minutes or more depending on the recipe and the type of hops. Brewers can use several different equations to figure out the amount of hops they need to get a certain amount of bitterness in the beer, commonly quantified as IBUs, or International Bittering Units.
Many variety of hops are grown to have high alpha acid levels to aid in the bittering of the beer. These varieties are often call bittering hops, and are used specifically for this purpose. Varieties used for bittering have a low oil content for reasons you will read later on.
Another very important aspect of alpha acids are there antiseptic nature. Before the usage of hops, high alcohol content was the only way to make beer last a long time. The addition of hops (especially the alpha acids) to beer helped prevent unwanted growth of bacteria (Gram positive), and on the other hand enhanced the ability of yeast to grow and ferment the wort to beer. No other place is there a better example of hops and preservation than the advent of the IPA. (What’s the story behind the IPA?)
Special care has to be taken with alpha acids though. If iso-alpha acids are allowed to react with light, and riboflavin (vitamin B2 coming from the malt), the beer will produce a “skunk” like smell that is not at all desired. This is why many beers are bottles in brown glass, to prevent certain wavelengths of light from starting this process.
As mentioned above, there are three main chemicals that the H. lupulus cone contributes to the brewing process:


Essential oils:
Essential oils are another key component of hop cones, adding aroma and flavor. Extracting the oils takes very little time when brewing beer. Due to their volatile nature, most of the initial oils from the starting hops (bittering hops) are evaporated off during the boil. The strength of the oils is seen in the last minutes of boiling (around 5 minutes), and in dry hopping. These oils produce most of the hop flavor and aroma smelled in beer. “Grassy”, “piney”, “earthy”, “citrus”, or “spicy” are all terms and fragrances that essential oils give to the beer.
Like the bittering hops with alpha acid, many varieties of hops are grown that produce a large amount of oils, and not a lot of alpha acids. These varieties are often called finishing hops due to them being added right at the end of the brewing process.
Some of the principle oils of the H. lupulus (making up roughly 60-80% of the oils in some varieties) are:
Humulene- Most of the flavor and aroma from this oil comes from adding it late in the boil, or dry hopping because it degrades very rapidly. This chemical is most notable in noble hops. Noble hops are variety of hops with high aroma (high levels of humulene), and low bitterness (low alpha acid): great hops for the last parts of brewing.
Myrcene- American hop varieties have higher amounts of this chemical. When added to the boil late or even after, myrcene creates a characteristic citrus and pine aroma in beer. Varieties high in this are used much in American dry hopped beers and American Craft beers. Although this chemical degrades fast, it helps in the final flavor of the beer.
Caryophllene- contributes a “spicy” flavor, similar to humulene.
Farensene- does not add much aroma or flavor to beer, as it is currently known. It makes up a small amount of the oils in the hop, but some varieties contain high amounts of this chemical.






Primary Purpose definitions:
Bittering- used in long boils for high alpha acid isomerisation (bitterness). Generally low oil content.
Finishing- used late in boil, after boil, or possibly dry hopping. High oil content, lower alpha acid content.
All-purpose- can be used for bittering or finishing.



Photo courtesy of Mark Witte,
beer lover and brewer
Hops after being used for dry hopping
Grape vs. Grain p. 97 Bamforth, 2008
Different hop varieties and characteristics of their aroma
The Brew-Master’s Bible p. 73 Snyder, 1997